NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
NCERT Solutions for Class 12 Chemistry Chapter 10 – Free PDF Download
According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 6.
NCERT Answers for Class 12 Science Chapter 10 Haloalkanes and Haloarenes given here have been made by the most recent schedule of the CBSE. The NCERT Answers for Class 12 Science chiefly comprises of replies to the activities and significant inquiries in the NCERT reading material. Allude to these answers for become familiar with the proper approaches to addressing and tackling such inquiries.
The NCERT Answers for Class 12 Science have been arranged and addressed by our subject specialists. These arrangements are available in basic language for simplicity of understanding. Chapter 10 Haloalkanes and Haloarenes of NCERT Answers for Class 12, is accessible as a PDF and can be unreservedly downloaded from here. Understudies can really utilize it anyplace and whenever as a secret weapon material.
Class 12 Chemistry NCERT Solutions Chapter 10 Haloalkanes and Haloarenes – Important Questions
10.1. Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl, or aryl halides:
(i)(CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)CI
(iii) CH3CH2C(CH3)2CH2I
(iv)(CH3)3CCH2CH(Br)C6H5
(v)CH3CH(CH3)CH(Br)CH3
(vi)CH3C(C2H5)2CH2Br
(vii)CH3C(Cl)(C2H5)CH2CH3
(viii)CH3CH=C(CI)CH2CH(CH3)2
(ix)CH3CH=CHC(Br)(CH3)2
(x)P-CIC6H4CH2CH(CH3)2
(xi)m-ClCH2C6H4CH2C(CH3)3
(xii)o-Br – C6H4CH (CH3)CH2CH3
Ans: (I) 2-Chloro-3methylbutane, 2° alkyl halide
(ii) 3-Chloro-4methyl hexane, 2° alkyl halide
(iii) 1 – Iodo-2,2-dimethylbutane, 1 ° alkyl halide
(iv) l-Bromo-3, 3-dimethyl – 1-phenylbutane, 2° benzylic halide
(v) 2-Bromo-3-methylbutane, 2° alkyl halide
(vi) 1-Bromo-2-ethyI-2-methylbutane, 1° alkyl halide
(vii)3-Chloro-3-methylpentane, 3° alkyl halide
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide
(ix)4-Bromo-4-methylpent-2-ene, allylic halide
(x)1-Chloro-4-(2-methylpropyl) benzene, aryl halide
(xi)1-Chloromethyl-3-(2,2-dimethylpropyl) benzene, 1 ° benzylic halide.
(xii)1-Bromo-2-(l-methylpropyl) benzene,aryl halide.
10.2. Give the IUPAC names of the following compounds:
(i) CH3CH(CI)CH (Br)CH3 (ii) CHF2CBrCIF (iii) CICH2C=CCH2Br (iv) (CCl3)3CCl
(v)CH3C(p-ClC6H4)2CH(Br)CH3 (vi)(CH3)3CCH=C(CI)C6H4I -p
Ans: (I) 2-Bromo-3-chlorobutane
(ii) 1 JBromo-1 – chloro-1,2,2-trifluoroethane
(iii) l-Bromo-4-chlorobut-2-yne
(iv)2-(Trichloromethyl)- l, 1,1,2,3,3,3-heptachloropropane
(v)2-Bromo-3,3-bis-(4-chlorophenyl) butane
(vi)l-Chloro-l-(4-iodophenyl)- 3,3-dimethylbut-l-ene.
10.3. Write the structures of the following organic halogen compounds:
(i)2-ChIoro-3-methylpentane
(ii)p-Bromochlorobenzene
(iii)l-Chloro-4-ethylcyclohexane
(iv)2r (2-Chlorophenyl) -1- iodooctane
(v)2-Bromobutane
(vi)4-tert-Butyl-3-iodoheptane
(vii)1-Bromo-4-sec-butyl-2-methylbenzene
(viii)1,4-Dibromobut-2-ene
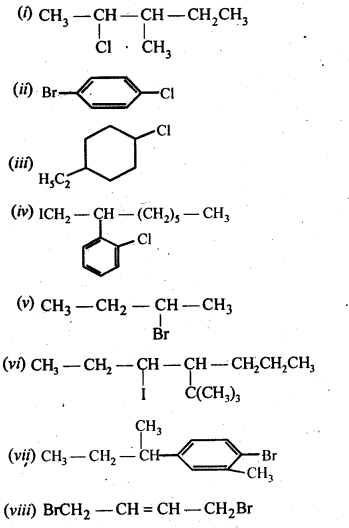
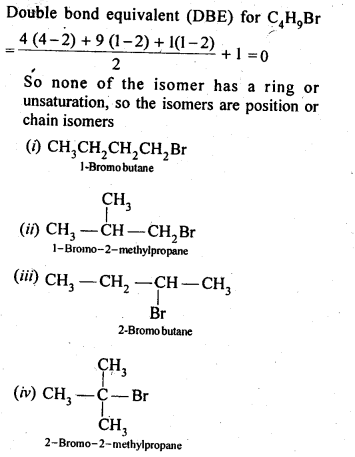
10.4. Write the isomers of the compound having formula C4H9Br.
10.5. Which compound in each of the following-pairs . will react faster in SN2 reaction with -OH? (i)CH3Br or CH3I
(ii)(CH3)3CCl or CH3Cl
Ans: (i)Since I-particle is an improved leaving bunch than Br-particle, thusly, CH3I responds quicker CH3Br in SN2 response with Goodness particle.
(ii)On steric grounds, 1° alkyl halides are more responsive than tert-alkyl halides in SN2 responses. Hence, CH3CI will respond at a quicker rate than (CH3)3CCl in a SN2 response with Gracious particle
10.6. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(I) 1-Bromo-l-methylcyclohexane
(ii) 2-Chloro-2-methylbutane.
(iii) 2,2,3-Trimethyl-3-bromopentane.

10.7. Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Ans: (I) sp2-half breed carbon in chlorobenzene is more electronegative than a sp3-crossover carbon in cyclohexylchloride, because of more prominent s-character. Hence, C iota of chlorobenzene tends to deliver electrons to Cl than carbon particle of cyclohexylchloride.
Therefore, C – Cl bond in chlorobenzene is less polar than in cyclohexylchloride. Further, because of delocalization of solitary sets of electrons of the Cl molecule over the benzene ring, C-Cl bond in chlorobenzene procures some twofold bond character while the C – Cl in cyclohexy! chloride is an unadulterated single security. As such, C-Cl bond in chlorobenzene is more limited than in cyclohexyl chloride.
Since dipole second is a result of charge and distance, subsequently, chlorobenzene has lower dipole second than cyclohexylchloride because of lower greatness of negative charge on the Cl iota and more limited C-Cl distance.
(ii) Alkyl halides are polar atoms, along these lines, their particles are kept intact by dipole fascination. The atoms of H2O are keep intact by H-bonds. Since the new powers of fascination among water and alkyl halide atoms are more vulnerable than the powers of fascination previously existing between alkyl halide – alkyl halide particles and water particles, thefefore, alkyl halides are immiscible (not solvent) in water. Alkyl halide are neither ready to frame H-bonds with water nor can break the H-bouncing organization of water.
(iii)Grignard reagents are extremely receptive. They respond with dampness present in the contraption to frame alkanes
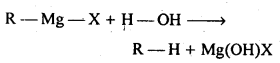
Thus, Grignard reagents must be prepared under anhydrous conditions.
