NCERT Solutions Class 12 Chemistry/Chapter 4 cChemical Kinetics
Chapter 4 Chemical Kinetics
NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics – Free PDF Download
NCERT Answers for Class 12 Chapter 4 Chemical Kinetics are the review materials that will help understudies in getting tuned in with the ideas associated with synthetic energy. The NCERT Answers for Class 12 Science PDF for compound energy are useful for the understudies of CBSE Class 12. These NCERT Arrangements are ready by subject specialists at Notesavailable.in as per the most recent CBSE Prospectus for 2023-24 in basic language for simple comprehension.
Understudies trying to make a vocation in the clinical or designing field should rehearse the NCERT Answers for Class 12 Science to score well in the board tests as well as different cutthroat selection tests. Further, these arrangements can likewise help understudies in planning notes of significant ideas or formulae. Understudies can get the free PDF of the NCERT Answers for Chapter 4 Chemical Kinetics by tapping the connection gave beneath.
Section Name | Topic Name |
4 | |
4.1 | Rate of a Chemical Reaction |
4.2 | Factors Influencing Rate of a Reaction |
4.3 | Integrated Rate Equations |
4.4 | Pseudo First Order Reaction |
4.5 | Temperature Dependence of the Rate of a Reaction |
4.6 | Collision Theory of Chemical Reactions |
NCERT TEXTBOOK QUESTIONS SOLVED
4.1) .For the reaction R—>P, the concentration of reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.

4.2.) In a reaction, 2A —-> Products, the concentration of A decreases from 0.5 mol L-1 to 0.4 molL-1 in 10 minutes. Calculate the rate during this interval?

4.3. For a reaction, A + B → Products, the rate law is given by : r = k [A]1/2[B]2. What is the order of reaction?
Ans. Rate law(r) = k [A]1/2[B]2
order of reaction = 12+2=212 or 2.5
4.4.) The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y ?
Ans. The reaction is : X—>Y
According to rate law,
rate = k[X]2
If [X] is increased to 3 times, then the new rate is
rate’ = k[3X]2
rate’ = 9 k [X]2 = 9 rate
Thus, rate of reaction becomes 9 times and hence rate of formation of Y increases 9-times.
4.5.) A first order reaction has a rate constant 1.15 x 10-3 s-1. How long will 5 g of this reactant take to reduce to 3 g?

4.6.Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Ans. For 1st order reaction,
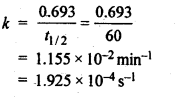
4.7.) What will be the effect of temperature on rate constant?
Ans. As a general rule, the rate steady for a response almost turns out to be twofold with around 10° climb in temperature in view of the way that the successful crashes become practically twofold. The specific reliance of the response rate on temperature is given by Arrhenius condition; k=Ae−Ea/Rt.
Where An is the Arrhenius factor or the recurrence factor. It is likewise called pre remarkable variable. It is a consistent intended for a specific response. R is gas consistent and Ea is actuation energy estimated in joules/mole (J mol-1).
NCERT EXERCISES
4.5.) Mention the factors which affect the rate of a chemical reaction.
Sol: The rates of chemical reactions are influenced by a number of factors. These are :
(i) Concentration of reactants. The pace of a substance response is relative to the grouping of the responding species participating in the response. It is greatest to begin with and gradually diminishes since the grouping of the responding species diminishes in like manner. In the event of reversible synthetic responses, the pace of substance response can be read up independently for both the forward and in reverse responses. In the event of vaporous responses, the expansion in pressure expands the response rate.
(ii) Temperature. As a rule, the expansion in temperature builds the response rate (there are a couple of exemptions too). As a matter of fact, the energy of the reactant species increments with the expansion in temperature thus will be number of crashes. It has been seen that in the majority of the cases, around 10° expansion in temperature makes response rate twofold. If it’s not too much trouble, note that the impact of temperature is very free of the convergence of the reactant species.
(iii) Presence of catalyst. In numerous compound responses, the response rate can be upgraded by specific unfamiliar substances called impetuses. These are really not consumed in the responses and furthermore donot go through any adjustment of substance attributes. Notwithstanding, their actual states, for example, variety, molecule size and so on, could change. Certain impetuses may have antagonistic impact as well as the response rate. They bring about diminishing the response rate as opposed to expanding it. These are called negative impetuses or inhibitors.
(iv) Nature of reactants. The idea of the responding species may likewise the impact the response rate. For instance, ignition of nitric oxide (NO) is quicker when contrasted with that of carbon monoxide (CO)
