NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds
NCERT Solutions for Class 12 Chemistry Chapter 9 – Free PDF Download
As indicated by the CBSE Schedule 2023-24, this section has been renumbered as Chapter 5.
NCERT Answers for Class 12 Science Chapter 9 Coordination Compounds introduced here assist understudies with finding out about “Coordination Compound” and comprehend the ideas connected with them. This section which is remembered for the Prospectus of CBSE Class 12 Science, has significant ideas as we discuss molecules and synthetic responses. Accordingly, Coordination Mixtures incorporate nitty gritty NCERT Solutions for Class 12 Chemistry for all the activity questions present in the section.
To comprehend the key points plainly to keep away from any trouble from here on out and score great imprints in the board assessment, understudies can allude to the NCERT Solutions for Class 12 Chemistry. Subject specialists at notesavailable.in have made these arrangements as per the most recent CBSE Prospectus 2023-24. Access the Science NCERT Answers for Chemistry NCERT Solutions for Class 12 PDF Chapter 9 by clicking the link below.
NCERT INTEXT QUESTIONS
9.1. Write the formulas for the following coordination compounds:
(i)Tetraamminediaquacobalt(IlI) chloride
(ii)Potassium tetracyanidonickelate(II)
(iii)Tris(ethanp-1,2-diamine) chromium(III) chloride
(iv)Amminebromidochloridonitrito-N- platinatc(II)
(v)Dichloridobis(ethane-l ,2-diamine) platinum (IV) nitrate
(vi)Iron(III)hexacyanidoferrate(II)
Ans: (i) [CO(NH3)4(H2O)2]Cl3.
(ii)K2[Ni(CN)4]
(iii)[Cr(en)3]Cl3
(iv)[Pt (NH3) Br Cl (N02)]–
(v)[PtCl2(en)2](N03)2
(vi)Fe4[Fe(CN)6]3
9.2. Write IUPAC names of following co-ordination compounds :
(a) [CO(NH3)6]Cl3
(b) [CO(NH3)Cl]Cl2
(C) K3[Fe(CN)6]
(d) [K3[Fe(C2O4)3]
(e) K2[PdCl4]
(f) [Pt(NH3)2ClNH2CH3]Cl.
Ans:
(a) hexaamminecobalt (III) chloride
(b) pentaamminechloridocobalt (III) chloride
(c) potassium hexacyanoferrate (III)
(d) potassium trioxalatoferrate (III)
(e) potassium tetrachloridoplatinum (II)
(f) diamminechlorido (methylamine) platinum(II) chloride.
9.3. Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square planar structure is diamagnetic and [NiCl4]2- ion with tetrahedral geometry is paramagnetic.
Ans: Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2. Nickel in this complex is in + 2 oxidation state. It achieves + 2 oxidation state by the loss of the two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CN– ion is a strong field, under its attacking influence, two unpaired electrons in the 3d orbitals pair up.
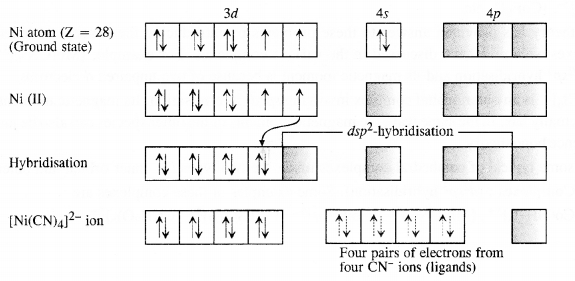
Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2 Nickel in this complex is in + 2 oxidation state. Nickel achieves + 2 oxidation state by the loss of two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CP ion is a weak field ligand, it is not in a position to cause electron pairing.
9.4. The hexaaquamanganese (II) ion contains five unpaired electrons while the hexacyano ion contains only one unpaired electron. Explain using crystal field theory.
Ans: Mn(II) particle has 3d5 design. Within the sight of H2O atoms going about as powerless field ligands, the dispersion of these five electrons is t32ge2 I. e., every one of the electrons stay unpaired to frame a high twist complex. Be that as it may, within the sight of CN-going about serious areas of strength for as ligands, the conveyance of these electrons is t52ge0g i.e., two t2g orbitals contain matched electrons while the third t2g orbital contains one unpaired electron. The complex framed is a low twist complex.
NCERT EXERCISES
9.1. Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans: The fundamental hypothesizes of Werner’s hypothesis of coordination compounds are as per the following:
(a)Metals have two kinds of valencies called
(I) essential valency which are ionisable; (ii) auxiliary valency which are non-ionisable
(b)Primary valency is fulfilled by the negative particles and it is what a metal shows in the development of its straightforward salts.
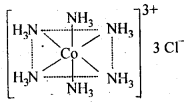
(c)Secondary valencies are fulfilled by unbiased ligand or negative ligand and are those which metal practices in the arrangement of its mind boggling particles. Each cation has a decent number of optional valencies which are coordinated in space about focal metal particle in specific fixed bearings, e.g„ In CoCl3-6NH3, valencies among Co and Cl are essential valencies and valencies among Co and NH3 are auxiliary. In COCl3-6NH3 , six smelling salts particles connected to Co by optional valencies are coordinated to six corners of a standard octahedron and in this manner represent design of COCl3-6NH3 as follows:
9.2. Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: Coordination substance: It is of a focal particle/particle clung to fixed number of particles or atoms by coordinate bonds for example [COCl3(NH3)3], [Ni (CO)4] and so on.
Ligand : The particles/particles bound to focal iota/particle in coordination element are called ligands. Ligands in above models are CL, NH3, CO Coordination number : This is the quantity of bond shaped by focal particle/particle with ligands. Coordination polyhedron : Spatial plan of ligands characterizing the state of complex. In above cases Co and Ni polyhedron are octahedral and tetrahedral in [CoCl3 (NH3)3] and [Ni(CO)4] separately.
Homoleptic : Metal is bound to just a single sort of ligands eg Ni in[Ni(CO)4]
Heteroletric : Metal is bound to more than one sort of ligandseg Coin [CoCl3(NH3)3]
9.3. Using IUPAC norms, write the formulae for the following
(a) tetrahydroxozincate(II)
(b) hexaammineplatinum (television)
(c) potassiumtetrachloridopalladate(II)
(d) tetrabromidocuprate (II)
(e) hexaaminecobalt(III) sulfate
(f) potassiumtetracyanonicklate (II)
(g) potassiumtrioxalatochromate(III)
(h) pentaamminenitrito-O-cobalt(III)
(I) diamminedichloridoplatinum(II)
(j) pentaamminenitrito-N-cobalt (III)
Ans: (a) [Zn(OH)4]2-
(b) [Pt(NH3)6]4+
(c) K2[PdCl4]
(d) [Cu(Br)4]2-
(e) [CO(NH3)6]2 (SO4)3
(f) K2[Ni(CN)4]
(g) K3 [Cr(OX)3]
(h) [CO(NH3)5ONO]2+
(I) [Pt(NH3)2Cl2]
(j) [CO(NH3)5NO2]2+.
9.4. Using IUPAC norms write the systematic names of the following:
(I) [Co(NH3)6]CI3,
(ii)[Pt(NH3)2CI (NH2CH3)] Cl
(iii) [Ti(H20)6]3+
(iv) [Co(NH3)4Cl(N02)]CI
(v)|Mn(H20)6]2+
(vi)[NiCl4]2-
(vii)[Ni(NH3)6]CI2
(viii)[Co(en)3]3+
(ix) [Ni(CO)4]
Ans: (I) Hexaammine cobalt (III) chloride.
(ii) Diammine chlorido (methylamine) platinum (II) chloride.
(iii) Hexaaquatitanium (III) particle.
(iv) Tetraammine chlorido nitrito-N-cobalt (IV) chloride.
(v)Hexaaquamanganese (II) particle.
(vi)Tetrachloridonickelate (II) particle.
(vii)Hexaammine nickel (II) chloride.
(viii)Tris (ethane – 1,2-diamine) cobalt (III) particle.
(ix) Tetra carbonyl nickel (0).
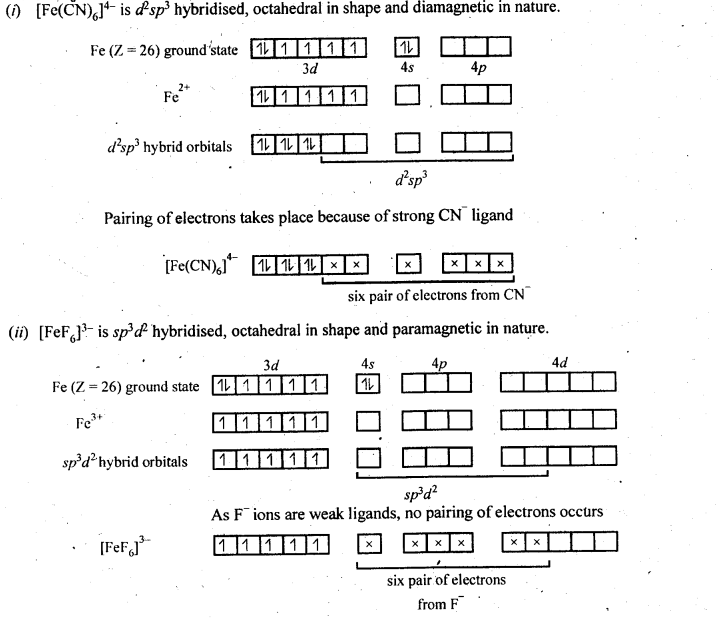
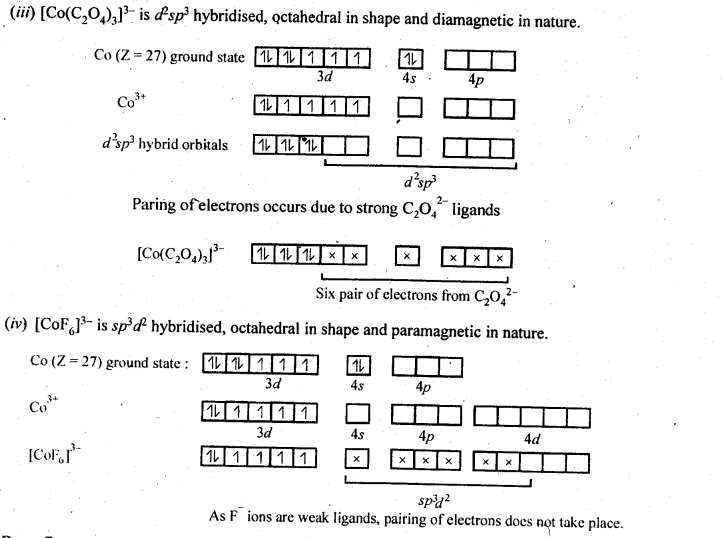
9.5. Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3-
(iv) [CoF6]3-
9.6. What is crystal field splitting energy? How does the magnitude of Δ0 decide the actual configuration of d-orbitals in a coordination entity?
Ans: When the ligands approach a progress metal particle, the d-orbitals split into two sets, one with lower energy and the other with higher energy. The distinction of energy between the two arrangements of orbitals is called gem field parting energy (Δ0 for octahedral field). If Δ0 < P (matching energy), the fourth electron enters
one of the e°g, orbitals giving the setup t32ge1g, in this way shaping high twist buildings. Such ligands for which Δ0 < P are called frail field ligands. In the event that Δ0 > P, the fourth electron joins together in one of the t2g orbitals giving the setup t42ge1g accordingly framing low twist edifices. Such ligands for which Δ0> P are serious areas of strength for called ligands.
9.7. A solution of [Ni(H20)6]2+ is green but a solution of [Ni(CN)4]2-is colourless. Explain.
Ans: In [Ni(H20)6]2+, Ni is in + 2 oxidation state and having 3d8 electronic design, in which there are two unpaired electrons which don’t coordinate in that frame of mind of the powerless H20 ligand. Consequently, it is hued. The d change retains red light and the integral light transmitted is green.
In [Ni(CN)4]2-Ni is additionally in + 2 oxidation state and having 3d8 electronic design. However, in presence of solid ligand CN-the two unpaired electrons in the 3d orbitals match up. Subsequently, there is no unpaired electron present. Consequently, it is boring.
9.8. [Fe(CN)6]4- and [Fe(H2O)6]2+ are of different colours in dilute solutions. Why?
Ans: In both the buildings, Fe is in + 2 oxidation state with d6 design. This implies that it has four unpaired electrons.Both CN-particle and H2O atoms which go about as ligands possess different relative situations in the spectrochemical series. They contrast in gem field parting energy (∆0). Clearly, they ingest radiations comparing to various frequencies/frequencies from the noticeable locale of light. (VIBGYOR) and the sent tones are additionally unique. This implies that the buildings have various tones in arrangements.
9.9. Discuss briefly giving an example in each case the role of coordination compounds in :
(a) biological systems,
(b) analytical chemistry,
(c) medicinal chemistry, and
(d) extraction/metallurgy of metals.
Ans:
(I) Coordination compounds are vital in organic frameworks. The color liable for photosynthesis, chlorophyll, is a coordination compound of magnesium. Hemoglobin, the red color 1 of blood which goes about as oxygen transporter is a coordination compound of iron. Vitamin B12, cyanocobalamine, the counter vindictive iron deficiency factor, is a coordination compound of cobalt. Among different mixtures of natural significance with composed metal particles are the compounds like, carboxypeptidase An and carbonic anhydrase (impetuses of organic frameworks).
(ii) There is developing Interest in the utilization of chelate treatment in restorative science. A model is the treatment of issues brought about by the presence of metals in harmful extents in plant/creature frameworks. Accordingly, abundance of copper and iron are taken out by the chelating ligands D-penicillamine and desferrioxime B by means of the arrangement of coordination compounds.
EDTA is utilized in the treatment of lead harming. Some coordination mixtures of platinum successfully hinder the development of growths. Models are: ds-platin and related compounds.
(iii) Coordination intensifies track down use in numerous subjective and quantitative substance examination. The natural variety responses given by metal particles with various ligands (particularly chelating ligands), because of development of coordination elements, structure the reason for their location and assessment by old style and instrumental techniques for investigation. Instances of such reagents incorporate EDTA, DMG (dimethylglyoxime), a-nitroso-β-naphthol, cupron, and so on.
(iv) Some significant extraction cycles of metals, similar to those of silver and gold, utilize complex development. Gold, for instance, joins with cyanide within the sight of oxygen and water to frame the coordination element [Au(CN)2]-in fluid arrangement. Gold can be isolated in metallic structure from this arrangement by the expansion of zinc.
